Bihar Board 12th Chemistry Objective Important Questions Part 1 in English
Bihar Board 12th Chemistry Objective Important Questions Part 1 in English
BSEB 12th Chemistry Objective Important Questions Part 1 in English
Question 1.
Number of σ and π Bonds in C2 Molecule is/are :
(a) 1σ and 1π
(b) 1σ and 2π
(c) 2π only
(d) 1σ and 3π
Answer:
(c) 2π only
Question 2.
The correct order of equivalent conductance of infinite dilution of LiCl, NaCl and KCl is :
(a) LiCl > NaCl > KCl
(b) KCl > NaCl > LiCl
(c) NaCl > KCl > LiCl
(d) LiCl > KCl > NaCl
Answer:
(b) KCl > NaCl > LiCl
Question 3.
The Molecule which has zero dipole moment is :
(a) NF3
(b) BF3
(c) CrO3
(d) CH2Cl2
Answer:
(b) BF3
Question 4.
A Dilute aqueous solution of’ sodium Fluoride is Electrolyzed the products at the anode and cathode are :
(a) F2, Na
(b) F2, H2
(c) O2, Na
(d) O2, H2
Answer:
(a) F2, Na
Question 5.
R-OH + CH2N2 → Leaving group in this reaction is :
(a) CH3
(b) R
(c) N2
(d) CH2
Answer:
(c) N2
Question 6.
The Product obtained when silica reacts with hydrogen Fluoride is :
(a) SiF4
(b) H2SiF6
(c) H2SiF4
(d) H2SiF3
Answer:
(a) SiF4
Question 7.
The Vant Hoffs factor of 0.1M Ba(NO3)2 solution is 2.74. The Degree of dissociation is:
(a) 91.3%
(b) 87%
(c) 100%
(d) 74%
Answer:
(b) 87%
Question 8.
Which of the following has the most stable +2 oxidation state :
(a) Sn
(b) Ag
(c) Fe
(d) Pb
Answer:
(d) Pb
Question 9.
Cannizzaro’s reaction is not given by :
(a) Formaldehyde
(b) Acetaldehyde
(c) Benzaldehyde
(d) Furfural
Answer:
(b) Acetaldehyde
Question 10.
Which Gas is absorbed strongly by charcoal :
(a) CO
(b) NH3
(c) NCl3
(d) H2
Answer:
(b) NH3
Question 11.
An organic compound gives Iodoform test and also gives positive test with Tollen reagent the compound is :
(a) CH3CHO
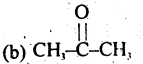
(c) CH3CH2OH
(d) (CH3)2CHOH
Answer:
(a) CH3CHO
Question 12.
Which Alkyl halide follows only SN2 hydrolysis Mechanism :
(a) CH3CH2-X
(b) (CH3)2CHX
(c) (CH3)3C-X
(d) C6H5CH2X
Answer:
(a) CH3CH2-X
Question 13.
The most stable oxidation states of Bismuth is :
(a) +3
(b) +5
(c) +3 and +5 Both
(d) None
Answer:
(a) +3
Question 14.
In Which of the following pairs of structure are tetrahedral as well as octahedral void respectively :
(a) B.C.C. and f.c.c.
(b) hcp and simple cubre
(c) hcp and ccp
(d) bcc and hcp
Answer:
(c) hcp and ccp
Question 15.
Which one of the following is Non-Crystalline or Amorphous :
(a) Diamond
(b) Graphite
(c) Glass
(d) Common salt
Answer:
(c) Glass
Question 16.
Which one of the following will produce maximum depression of freezing point:
(a) K2SO4
(b) NaCl
(c) Urea
(d) Glucose
Answer:
(a) K2SO4
Question 17.
If 2 gram of NaOH is present in 200 ml of its solution. Its molarity will be:
(a) 0.25
(b) 0.5
(c) 5
(d) 10
Answer:
(a) 0.25
Question 18.
Fused NaCl on Electrolysis gives on Cathode :
(a) Chlorine
(b) Sodium
(c) Sodium amalgam
(d) Hydrogen
Answer:
(b) Sodium
Question 19.
If 96500 Coulomb of Electricity is passed through CuSO4 solution it will liberate :
(a) 63.5 gm Cu
(b) 31.76 gm Cu
(c) 96500 gm Cu
(d) 100 gm Cu
Answer:
(b) 31.76 gm Cu
Question 20.
If the Rate of a reaction is expressed by-
Rate = K [A]2 [B] the order of reaction will be :
(a) 2
(b) 3
(c) 1
(d) 0
Answer:
(b) 3
Question 21.
Which one of the following Elements is found in Free state in Nature ?
(a) Sodium
(b) Iron
(c) Zinc
(d) Gold
Answer:
(d) Gold
Question 22.
Which one of the following does not form hydrogen-bonding ?
(a) NH3
(b) H2O
(c) HCl
(d) HF
Answer:
(c) HCl
Question 23.
Main source of Heliumis :
(a) Air
(b) Radium
(c) Monazite
(d) Water
Answer:
(c) Monazite
Question 24.
Which one of the following is diamagnetic ion :
(a) Co2+
(b) Ni2+
(c) Cu2+
(d) Zn2+
Answer:
(d) Zn2+
Question 25.
Antiferro magnetic oxide is :
(a) MnO2
(b) TiO2
(c) VO2
(d) CrO2
Answer:
(d) CrO2
Question 26.
Milk is an example of :
(a) Emulsion
(b) Suspension
(c) beam
(d) sol
Answer:
(a) Emulsion
Question 27.
Which of the following solution (in water) has highest boiling point:
(a) 1 M NaCl
(b) 1 M MgCl2
(c) 1 M Urea
(d) 1 M Glucose
Answer:
(b) 1 M MgCl2
Question 28.
Which of the following is Natural fibre :
(a) Starch
(b) Cellulose
(c) Rubber
(d) Nalon-6
Answer:
(b) Cellulose
Question 29.
Number of atoms in b.c.c unit cell is :
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(b) 2
Question 30.
Number of atoms in simple cubic unit cell is :
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(a) 1
Question 31.
Number of atoms in f.c.c. unit cell is :
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(d) 4
Question 32.
Number of Tetrahederal voids in per unit cell of f.c.c. structure contain same atoms :
(a) 4
(b) 6
(c) 8
(d) 12
Answer:
(c) 8
Question 33.
5 gram Glucose dissolved in 20 gram water, What percentage mass of solution :
(a) 25%
(b) 20%
(c) 5%
(d) 4%
Answer:
(b) 20%
Question 34.
The property of the solution does not depend on Temperature :
(a) Molarity
(b) Molality
(c) Normality
(d) Density
Answer:
(b) Molality
Question 35.
What is molality of water at 25°C is :
(a) 18 Mole Litre-1
(b) 10-7 Mole Litre-1
(c) 55.5 Mole Litre-1
(d) None of these
Answer:
(c) 55.5 Mole Litre-1
Question 36.
Buna-s is :
(a) Natural polymer
(b) Synthetic polymer
(c) Sulphur polymer
(d) None of these
Answer:
(b) Synthetic polymer
Question 37.
Nylon 66 is polyamide of:
(a) Vinyl chloride and formaldehyde
(b) adipic acid and methylamine
(c) adipic acid and hexamethylene diamine
(d) formaldehyde and melamine
Answer:
(c) adipic acid and hexamethylene diamine
Question 38.
Monomer of PVC is :
(a) Ethylene
(b) Tetrafluoroethylene
(c) Chloroethene
(d) None of these
Answer:
(c) Chloroethene
Question 39.
The function of flux during smelting of the ore is :
(a) To make the ore porous
(b) To facilitate reduction
(c) To remove gangue
(d) To facilitate oxidation
Answer:
(c) To remove gangue
Question 40.
Froth floatation process is based on :
(a) specific gravity of the ore particles
(b) Magnetic properties of the ore particles
(c) Electrical properties of the ore particles
(d) wetting properties of the ore particles
Answer:
(d) wetting properties of the ore particles
Question 41.
Carbon monoxide reduction process is used for the extraction of:
(a) Sn
(b) Ag
(c) Cu
(d) Fe
Answer:
(d) Fe
Question 42.
Argentite is a mineral of:
(a) Cu
(b) Ag
(c) Au
(d) Pt
Answer:
(b) Ag
Question 43.
Heating of pyrites to remove sulphur is called as :
(a) Roasting
(b) Smelting
(c) Calcination
(d) Fluxing
Answer:
(a) Roasting
Question 44.
Wood spirit is known as :
(a) Methanol
(b) Ethanol
(c) Acetone
(d) Acetic acid
Answer:
(a) Methanol
Question 45.
Which of the following does not reduces Fehling’s solution?
(a) Formic acid
(b) Acetic acid
(c) Acetaldehyde
(d) Formaldehyde
Answer:
(b) Acetic acid
Question 46.
Intermolecular hydrogen bonding is strongest in :
(a) Methylamine
(b) Phenol
(c) Methanol
(d) Formaldehyde
Answer:
(c) Methanol
Question 47.
Which transition metal can show highest oxidation state?
(a) Sc
(b) Ti
(c) Os
(d) Zn
Answer:
(c) Os
Question 48.
The catalyst used in Haber’s process is :
(a) tungsten
(b) molybdenum.
(c) Chromium
(d) iron containing molybdenum
Answer:
(d) iron containing molybdenum
Question 49.
In KMnO4, oxidation number of Mn is:
(a) +2
(b) +4
(c) +6
(d) +7
Answer:
(d) +7
Question 50.
Which of the following oxides is amphoteric in nature?
(a) NiO
(b) ZnO
(c) FeO
(d) CaO
Answer:
(b) ZnO
Question 51.
Natural rubber is a polymer of:
(a) Butadiene
(b) Ethylene
(c) Styrene
(d) Isoprene
Answer:
(d) Isoprene
Question 52.
Which of the following is a natural fibre?
(a) starch
(b) cellulose
(c) Rubber
(d) Nylon-6
Answer:
(b) cellulose
Question 53.
Which of the following is a polyamide?
(a) Teflon
(b) Nylon-6, 6
(c) Terylene
(d) Bakelite
Answer:
(b) Nylon-6, 6
Question 54.
In the colloidal state the particle size is :
(a) below 1 nm
(b) between 1 nm to 100 nm
(c) more than 100 nm
(d) none of the above
Answer:
(b) between 1 nm to 100 nm
Question 55.
Colloids can be purifled by :
(a) dialysis
(b) peptization
(c) coagulation
(d) condensation
Answer:
(a) dialysis
Question 56.
Which of the following is an antihistamine drug?
(a) Chloramphenicol
(b) Chloropheniramine maleate
(p) Ciprofloxacin
(d) Chloroquine
Answer:
(b) Chloropheniramine maleate
Question 57.
Which of the following drugs reduces fiver?
(a) Analgesic
(b) Antipyretic
(c) Antibiotic
(d) Tranquillizer
Answer:
(b) Antipyretic
Question 58.
Barbituric acid is used as :
(a) an antiseptic
(b) an antibiotic
(c) an analgesic
(d) a tranquillizer
Answer:
(d) a tranquillizer
Question 59.
Which of the following solutions have highest freezing point?
(a) 0.1 M Nacl
(b) 0.1M MgCl2
(c) 0.1 M Al2(SO4)3
(d) 0.1 M urea
Answer:
(d) 0.1 M urea
Question 60.
Which of the following solutions has the highest osmotic pressure?
(a) 1M NaCl
(b) 1m MgCl2
(c) 1M urea
(d) 1m glucose
Answer:
(b) 1m MgCl2
Question 61.
Which of the following solution (in water) has the highest boiling point?
(a) 1M NaCl
(b) 1M mgCl2
(c) 1M urea
(d) 1M glucose
Answer:
(b) 1M mgCl2
Question 62.
The add which forms two series of salts is :
(a) H3PO4
(b) H3PO3
(c) H3BO3
(d) H3PO2
Answer:
(b) H3PO3
Question 63.
Cinnabar is the ore containing :
(a) Ag2S
(b) HgS
(c) ZnS
(d) CuS
Answer:
(b) HgS
Question 64.
Which of the following solutions in water has highest boiling point:
(a) 1M HCl
(b) 1M MgCl2
(c) 1M urea
(d) 1M Glucose
Answer:
(b) 1M MgCl2
Question 65.
Osmotic pressure of the solution can be increase by :
(a) Increasing temperature of the solution
(b) Decreasing temperature of the solution
(c) Increasing the volume of the vessel.
(d) Diluting the solution
Answer:
(a) Increasing temperature of the solution
Question 66.
Leaching is a process of:
(a) Reduction
(b) Concentration
(c) Refining
(d) Oxidation
Answer:
(b) Concentration
Question 67.
In the manufacture of iron form haematite, limestone is added to act as :
(a) A reducing agent
(b) Proxidising agent
(c) Flux
(d) Slag
Answer:
(c) Flux
Question 68.
Zone refining is a method to obtain :
(a) Very high temperature
(b) Ultrapure metals
(c) Ultrapure Al
(d) Ultrapure oxides
Answer:
(b) Ultrapure metals
Question 69.
The common method of extraction of metal from oxide ores is :
(a) Reduction with carbon
(b) Reduction with hydrogen
(C) Reduction with Aluminium
(d) Electrolytic method
Answer:
(a) Reduction with carbon
Question 70.
Which oil is used as further in froth floatation process?
(a) Mustard oil
(b) Olive oil
(c) Coconut oil
(d) Pine oil
Answer:
(d) Pine oil
Question 71.
When phenol is distilled with zinc dust it gives :
(a) Benzene
(b) Toluene
(c) Benzaldehyde
(d) Benzoic acid
Answer:
(a) Benzene
Question 72.
Backlste is:
(a) Addition polymer
(b) Elastomer
(c) Thermoplastic
(d) Thermosetting
Answer:
(d) Thermosetting
Question 73.
Which of the following polymer is a copolymer?
(a) polyprene
(b) nylon-66
(c) Terylene
(d) Neoprene
Answer:
(b) nylon-66
Question 74.
Caprolactam is the monomer for :
(a) Nylon-6
(b) Terylene
(c) Nylone-66
(d) Nylon-6/0
Answer:
(c) Nylone-66
Question 75.
The Tyndall effect associated with colloidal particles is due to :
(a) presence of electrical charge
(b) Scattering of light
(c) absorption of light
(d) reflection of light
Answer:
(b) Scattering of light
Question 76.
Which of the following is a tranquillizer?
(a) Seconal
(b) Morph in
(c) Streptomycin
(d) Phenacetin
Answer:
(a) Seconal
Question 77.
Which of the following is used as a preservative to protect processed food?
(a) Sodium sulphate
(b) Saccharin
(c) BHT
(d) Sodium metabisulphate
Answer:
(d) Sodium metabisulphate
Question 78.
Which of the following is used for hypertension?
(a) antacid
(b) paracetamol
(c) aspirin
(d) reserpine
Answer:
(d) reserpine
Question 79.
Which of the following can be used as an artificial sweetener?
(a) equanil
(b) sucrose
(c) sucralose
(d) glucose
Answer:
(c) sucralose
Question 80.
Which of the following in antibacterial?
(a) penicillin
(b) sulphapyridine
(c) ciprofloxacine
(d) all
Answer:
(d) all
Question 81.
Which of the following is a colligative property?
(a) surface tension
(b) viscosity
(c) osmotic pressure
(d) concentration
Answer:
(c) osmotic pressure
Question 82.
For the first order reaction, the half-life is independent of:
(a) catalyst
(b) temperature
(c) both catalyst and temperature
(d) initial concentration of reactants
Answer:
(d) initial concentration of reactants
Question 83.
The rate of a reaction depends upon :
(a) temperature
(b) time
(c) initial concentration
(d) none
Answer:
(a) temperature
Question 84.
Which of the following is a lyophobic colloids?
(a) gelatin
(b) sulphur
(c) starch
(d) gum arabic
Answer:
(b) sulphur
Question 85.
Which of the following is a hydrophobic (lyophobic) sol?
(a) starch solution
(b) gum solution
(c) protein solution
(d) arsenous sulphide sol.
Answer:
(d) arsenous sulphide sol.
Question 86.
Which one of the following is a hydrophilic (lyophilic) colloidal sol?
(a) Barium sulphate sol.
(b) Arsenic sulphide sol.
(c) Starch solution
(d) Silver chloride sol.
Answer:
(c) Starch solution
Question 87.
Colloidal particles in a sol can be coagulated by :
(a) heating
(b) adding an electrolyte
(c) adding oppositely charged sol.
(d) any of the above method
Answer:
(d) any of the above method
Question 88.
Emiilsibier is an agent which :
(a) accelerates the dispersion
(b) homogenises an emulsion
(c) stabilises an emulsion
(d) aids the flocculation of an emulsion
Answer:
(c) stabilises an emulsion
Question 89.
Milk is an emulsion of fat, etc dispersed in :
(a) water
(b) amino acids
(c) saline water
(d) fatty acids
Answer:
(a) water
Question 90.
Blood may be purified by :
(a) dialysis
(b) Coagulation
(c) electro-osmosis
(d) filteration
Answer:
(a) dialysis
Question 91.
Which of the following oxides is the most acidic?
(a) N2O5
(b) P2O5
(c) AS2O5
(d) Sb2O5
Answer:
(a) N2O5
Question 92.
Which of the following would be diamagnetic?
(a) Cu2+
(b) Ni2+
(c) Ca2+
(d) Ti3+
Answer:
(c) Ca2+
Question 93.
Which of the following would be paramagnetic
(a) Zn2+
(b) Cu+
(c) Sc3+
(d) Mn2+
Answer:
(d) Mn2+
Question 94.
The oxidation state of Cr in [Cr (NH3)4 Cl2]+ is :
(a) +3
(b) +2
(c) +1
(d) 0
Answer:
(a) +3
Question 95.
An amorphous solid is :
(a) NaCl
(b) CsCl
(c) Tar
(d) CaF2
Answer:
(c) Tar
Question 96.
Which one has highest melting point?
(a) ionic crystal
(b) molecular crystal
(c) covalent crystal
(d) metallic crystal
Answer:
(a) ionic crystal
Question 97.
Bravais lattices are of:
(a) 10 types
(b) 8 types
(c) 7 types
(d) 14 types
Answer:
(d) 14 types
Question 98.
NaCl crystal belongs to the crystal system :
(a) tetragonal
(b) cubic
(c) orthorhombic
(d) monoclinic
Answer:
(b) cubic
Question 99.
The coordination number of each atom in bee is :
(a) 4
(b) 6
(c) 8
(d) 12
Answer:
(c) 8
Question 100.
In a face centred cubic lattice the number of nearest neighbours for a given lattice point is :
(a) 6
(b) 8
(c) 12
(d) 14
Answer:
(c) 12
Question 101.
When a lead storage battery is charged, it acts as :
(a) a primary cell
(b) an electrolytic cell
(c) a galvanic cell
(d) a concentration cell
Answer:
(b) an electrolytic cell
Question 102.
Which of the following metals can deposit copper from copper sulphate?
(a) mercury
(b) platinum
(c) gold
(d) iron
Answer:
(d) iron
Question 103.
The unit of specific conductivity is :
(a) ohms cm-1
(b) ohms-1 cm-1
(c) ohms-1cm
(d) ohms-1cm-2
Answer:
(b) ohms-1 cm-1
Question 104.
The rate constant of a reaction increases with increase of temperature because :
(a) the activation energy increases
(b) the number of activated molecules decreases
(c) the activation energy decreases
(d) the number of activated molecules increases
Answer:
(d) the number of activated molecules increases
Question 105.
The rate constant of a reaction depends upon :
(a) temperature
(b) pressure
(c) volume
(d) all the three
Answer:
(a) temperature
Question 106.
Laughing gas is:
(a) NO
(b) NO2
(c) N2O
(d) N2O5
Answer:
(c) N2O
Question 107.
Phosphorus is kept in
(a) kerosene oil
(b) water
(c) alcohol
(d) ammonia
Answer:
(b) water
Question 108.
Structure of ammonia is :
(a) trigonal
(b) tetrahedral
(c) pyramidal
(d) trigonal pyramid
Answer:
(c) pyramidal
Question 109.
Which one of the acids is a dibasic acid?
(a) H3PO3
(b) HPO3
(c) H3PO2
(d) H3PO4
Answer:
(a) H3PO3
Question 110.
When ammonia is passed over heated CuO it is oxidised to :
(a) HNO2
(b) N2O
(c) N2
(d) NO2
Answer:
(c) N2
Question 111.
The most stable allotropic form of sulphur is :
(a) rhombic
(b) monoclinic
(c) plastic
(d) milk of sulphur
Answer:
(a) rhombic
Question 112.
Which Shows maximum catenation property?
(a) Te
(b) Se
(c) S
(d) O
Answer:
(c) S
Question 113.
The formula of oleum is :
(a ) H2SO5
(b) H2S2O7
(c) H2S2O3
(d) H2S2O8
Answer:
(b) H2S2O7
Question 114.
Ozone molecule is :
(a) Linear
(b) triangular
(c) tetrahedral
(d) none of these
Answer:
(b) triangular